
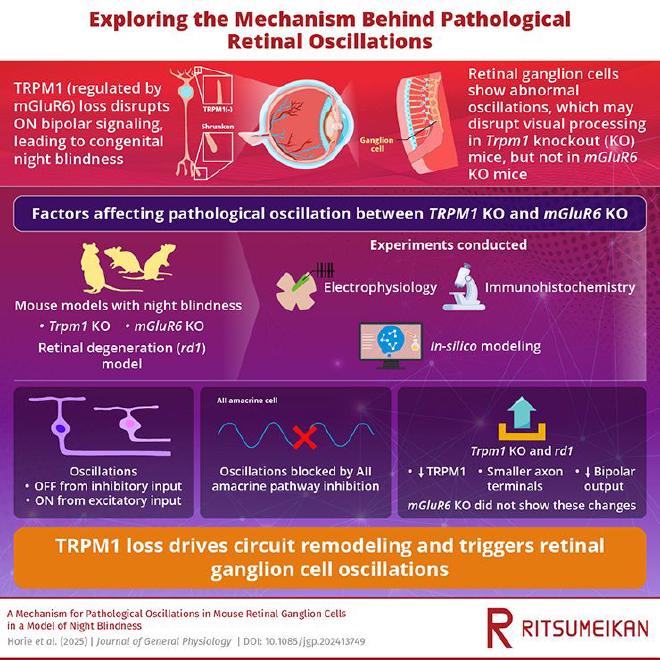
In a recent study published online in The Journal of General Physiology, led by Mr. Sho Horie, a PhD candidate, from the Graduate School of Pharmacy, Ritsumeikan University, Japan, along with Professor Katsunori Kitano, Professor Masao Tachibana, and Professor Chieko Koike, from Center for Systems Vision Science, Ritsumeikan University, revealed that the loss of a single ion channel—TRPM1—sets off a cascade of changes that lead to persistent oscillations in the retina. Their findings not only illuminate the cellular basis of CSNB but also identify a common mechanism underlying retinal degenerative conditions,** such as RP.
“Most of the phenotypes of the respective gene knockout mice are coincidental, but only the Trpm1 knockout (KO) mouse retina has spontaneous oscillation. Hence, we tried to figure out the difference between Trpm1 and mGluR6 KO mice,” explained Mr. Horie.
Using whole-cell clamp recordings and computational modeling, the team examined how TRPM1 loss alters retinal signaling. They found that in Trpm1 KO mice, inhibitory and excitatory inputs to RGCs oscillate in opposite phases, creating anti-phase rhythmic activity between OFF and ON pathways. Blocking specific synaptic and gap junction pathways silenced these oscillations, pinpointing the source to a disrupted circuit involving rod bipolar cells (RBCs) and AII amacrine cells (ACs).
“Under certain pathological conditions, RGCs can display spontaneous oscillatory activity,” noted Professor Koike. “This ‘noise’ disrupts visual information processing and can cause hallucinations. Our study reveals why such oscillations occur in Trpm1 KO mice and suggests that the same mechanism drives them in degenerative diseases like RP.”
The researchers were able to replicate the oscillatory firing patterns seen experimentally by incorporating these structural and electrical changes into a computational model. The model confirmed that reduced synaptic strength between RBCs and ACs, combined with hyperpolarization of ON bipolar cells, is sufficient to trigger pathological rhythmic firing.
Professor Kitano added: “Our simulations show that even small reductions in bipolar cell output can destabilize retinal circuits, leading to oscillations that mask real visual signals.”
The team hopes their findings will pave the way for new therapeutic approaches to stabilize retinal activity and improve outcomes in vision restoration treatments.
Citation #
- The study A mechanism for pathological oscillations in mouse retinal ganglion cells in a model of night blindness was published in the Journal of General Physiology
Funding #
This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grants Nos. 24H00747, 22KK0137, 19H01140, and 24390019), Takeda Science Foundation, the Kobayashi Foundation, JST PRESTO, and R-GIRO.
Contact [Notaspampeanas](mailto: notaspampeanas@gmail.com)

