
The model could also be the key to environmentally friendly fuels, as researchers are working on imitating natural photosynthesis and using sunlight to produce high-energy compounds: solar fuels such as hydrogen, methanol and synthetic petrol. If burned, they would produce only as much carbon dioxide as was needed to produce the fuels. In other words, they would be carbon-neutral.
A molecule with a special structure #
In the scientific journal Nature Chemistry, Professor Oliver Wenger and his doctoral student Mathis Brändlin have now reported on an important interim step toward achieving this vision of artificial photosynthesis: they have developed a special molecule that can store four charges simultaneously under light irradiation – two positive ones and two negative ones.
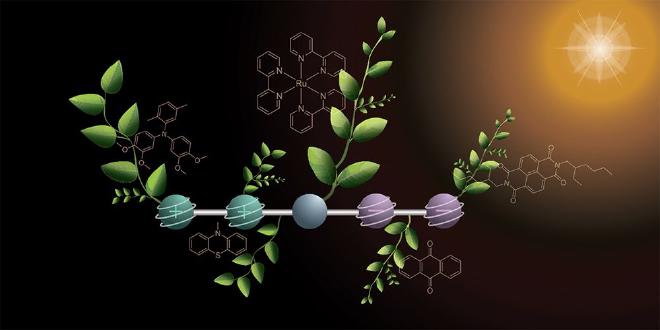
The molecule consists of five parts that are linked in a series and each performs a specific task. One side of the molecule has two parts that release electrons and are positively charged in the process. Two on the other side pick up the electrons, which causes them to become negatively charged. In the middle, the chemists placed a component that captures sunlight and starts the reaction (electron transfer).
Two steps using light #
In order to generate the four charges, the researchers took a stepwise approach using two flashes of light. The first flash of light hits the molecule and triggers a reaction in which a positive and a negative charge are generated. These charges travel outward to the opposite ends of the molecule. With the second flash of light, the same reaction occurs again, so that the molecule then contains two positive and two negative charges.
Works in dim light #
“This stepwise excitation makes it possible to use significantly dimmer light. As a result, we are already moving close to the intensity of sunlight,” explained Brändlin. Earlier research required extremely strong laser light, which was a far cry from the vision of artificial photosynthesis. “In addition, the charges in the molecule remain stable long enough to be used for further chemical reactions.”
A tiny step… with huge possibilities #

Citation #
-
The paper Photoinduced Double Charge Accumulation in a Molecular Compound was published in the Nature Chemistry journal. Authors: Mathis Brändlin, Björn Pfund, Oliver S. Wenger.
-
The article Chemists develop molecule for important step toward artificial photosynthesis was published in the UNIBAS’s English news section
Contact [Notaspampeanas](mailto: notaspampeanas@gmail.com)

