In an article in NIH, written by Sharon Reynolds, you can red that ‘cancer arises from normal cells in the body that morph into something else entirely. Changes to their DNA sequence, called mutations, can give cells the ability to grow into a tumor. Some cells may spread, sometimes fatally, to other parts of the body. But the genetic changes that drive cancer cells also mark these dangerous invaders, like a barcode on a package’.
Cancer cells and identifying pieces of material from them can make their way into the blood and other fluids, like urine. Over the last decade, scientists have made huge strides in finding and using these clues in bodily fluids. This strategy is often called a liquid biopsy. Liquid biopsies can be both less invasive and more informative than taking a sample of tumor tissue itself.
Liquid biopsy tests have been used successfully for years to select people to receive certain targeted cancer therapies. Researchers are now working to integrate liquid biopsies in other aspects of cancer care, explained Dr. Christos Patriotis, a cancer prevention expert at NIH. “They’re being tested for everything from screening and early detection all the way to guiding treatment.”
And the field has been advancing rapidly, Patriotis explained. “We hope that in the next three, four, five years, liquid biopsies will start becoming routine in the clinic,” he said.
Signs in the blood #
The first liquid biopsies aimed to count the number of tumor cells in the bloodstream. “People were really excited that you could get this information out of the blood,” said Dr. Brian Sorg, a liquid biopsy expert at NIH. “But they quickly found out that just counting circulating tumor cells doesn’t give you that much information about a tumor.”
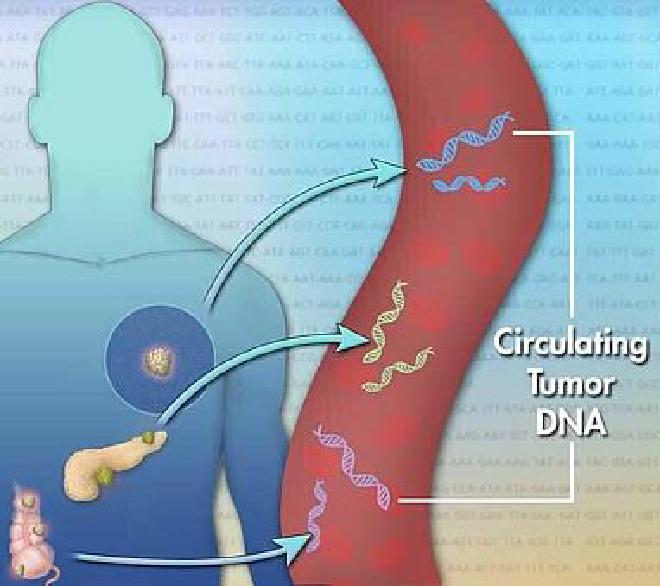
Over the last decade, researchers have switched their focus to the material that spills out of tumor cells—such as DNA, RNA, proteins, and more. Much of this work has concentrated on circulating tumor DNA (ctDNA).
Myriad studies have shown that, for tracking a known tumor during or after treatment, looking for single mutations in ctDNA works well. But in looking for evidence of whether a cancer exists, such as in aiding an initial diagnosis, it can fall short. Research teams **have started looking for ways to amplify faint ctDNA signals. One approach entails injecting substances into the blood ahead of a liquid biopsy to help capture the precious genetic material.
But researchers have been delving into information that may be more plentiful or informative than single mutations.
Copy number alteration #
For example, in a recent study, researchers measured a type of gene change called a copy number alteration in blood samples from children with a variety of cancer types. Copy number alterations are when pieces of chromosomes get deleted or duplicated. The test successfully picked out 70% of the kids with cancer. In some, the alterations could no longer be detected following treatment. This suggests that such tests might be used to monitor patients after treatment.
CNVs can be large-scale duplications or deletions that affect a whole exon, gene, or even several genes.
On the other hand, CNA specifically refers to deletions or amplifications of fragments of genomic material, which are particularly common in cancer and play a significant role in its development and progression.
While CNVs are generally considered to be inherited or present in the germline, CNAs are often somatic, occurring in tumor cells.
Therefore, while CNVs and CNAs both involve changes in the number of copies of a genomic region, they are not exactly the same, with CNVs being a broader term that includes both germline and somatic variations, and CNAs focusing more on the somatic changes seen in cancer.
Sources: Wikipedia; ScienceDirect; PubMed.NCBI.NLM.NIH.gov; Genome.gov; Karger & Nature
Other recent studies tested whether measuring chemical tags called methylation patterns found in genetic material in the blood could pick out cancer. Methylation patterns are known to differ between normal and cancerous cells. Researchers found that such patterns in liquid biopsies could be used to detect cancer. For example, one blood test had a 99% chance of picking out people with kidney cancer, including early-stage kidney cancer, from those without.
Promisingly, blood tests using methylation patterns have even been able to distinguish between different types of brain tumors. To diagnose a brain tumor this way, “you may have to use cerebrospinal fluid instead of blood,” said Patriotis, “but that’s still so much easier on patients than going into the brain itself.”
Work is ongoing to adapt these kinds of liquid biopsy tests to use urine samples, which are even easier to collect than blood. _Tests that use saliva may also be able to pick up cancer types far distant from the mouth**, Sorg explained. “For reasons we don’t yet understand, a lot of things tend to end up in saliva from elsewhere in the body,” he said.
Beyond DNA #
Dr. Ajay Goel, a biomarker researcher at City of Hope, an NIH Comprehensive Cancer Center, has been studying pieces of genetic material that are more abundant than ctDNA. These include microRNA, which helps carry messages from cell to cell in the body.
“Every cancer cell has a single copy of its DNA. But the same tumor cell is releasing thousands of copies of microRNAs and other RNA molecules, making them easy to find,” Goel explained. MicroRNAs come with the added advantage of being packed in protective bubbles called extracellular vesicles to pass though the bloodstream. Extracellular vesicles contain markers on them that tell exactly where in the body they came from.
“These markers are a zip code for every tissue type in the body, and they’re easily trackable in the bloodstream or other bodily fluids,” he said.
Knowing where a marker of cancer is coming from in the body is vital if people want to find tumors early, not just track them after a diagnosis.
Pancreatic cancers #
Goel and his team have been testing a microRNA-based liquid biopsy to screen people at high risk for pancreatic cancer. Currently, most pancreatic cancers are found at an advanced stage, when they’re almost universally fatal. With liquid-biopsy based testing, more cancers might be detected when they could still be removed.
“Right now, only about 10 to 15% of people have disease that can be removed with surgery,” Goel says. “Our goal is to get that up to 50%, maybe even 80 or 90% one day.”
In a recent study on blood samples from several hundred people with early-stage pancreatic cancer and people without cancer, their microRNA-based liquid biopsy successfully picked out almost all of the people with cancer. The team is now running a study in people at high risk for pancreatic cancer to see if the test can catch tumors early, before symptoms develop.
Goel’s team has also been testing other potential biomarkers to include in liquid biopsies. “In the end, the best liquid biopsy is probably going to be some kind of cocktail, some combination of biomarkers, whether it’s RNA, DNA, or proteins—all of them have something to offer to get the highest possible combination of sensitivity and specificity,” Goel said.
Multi-cancer detection #
Sensitivity and specificity are both crucial for the ultimate hope for liquid biopsies: multi-cancer detection (MCD) tests. MCD tests could potentially be used to screen healthy people for dozens of cancer types at once.
The sensitivity of a test refers to its ability to find a disease in people who actually have that disease. Missing a case of cancer—a failure of sensitivity—is called a false-negative result. Specificity refers to a test’s ability to identify who doesn’t have cancer. A failure of specificity is called a false-positive result—that can result in telling someone they have a disease when they don’t.
For an MCD test to be safe for widespread use, Patriotis explained, it would have to produce almost zero false positive or negative results. Currently available MCD test aren’t there yet, even though some are already being directly marketed to consumers. No studies to date have shown they can reduce the risk of death from any cancer type. “At the moment, the harms might be greater than the benefits,” Patriotis admitted.
CancerSEEK #
But these tests do have great promise. NIH funding is helping to spur the development of many such tests. A notable one is called CancerSEEK. In a large clinical trial, the test found tumors for which no screening tests currently exist, such as ovarian and uterine cancers. But it also produced false-positive results, with many participants receiving unnecessary follow-up tests, some of which were invasive.
In addition to improving the accuracy of MCD tests, explained Patriotis, other issues also need to be resolved. For example, these tests may find hints of tumors so small they can’t even be located on follow-up imaging tests. When it comes to that sort of scenario, he says, “There are no guidelines for physicians about what to do next.”
To help address some of these questions, NIH plans to launch a large study called the Vanguard Study on Multi-Cancer Detection. Clinical trials resulting from that study will hopefully help determine if MCD tests can reduce illness and death from cancer and, if so, provide guidance for doctors on how to use them.
All we have is hope, and in some deeper instance, faith… and science.
“Can we use these non-invasive approaches in average-risk people with no symptoms, who are brewing cancer in their body? Can we find those cancers early enough to intercept the disease and extend survival? I think that’s where the biggest opportunity lies ahead of us,” Goel said.
Treatment guidance #
While the use of liquid biopsies for cancer screening remains a hope for now, their use in cancer treatment has been growing. Liquid biopsies are crucial for helping to guide the use of targeted therapies. Such drugs shut down the cellular machinery driven by certain genetic mutations.
Targeted drugs have revolutionized the treatment of many cancer types over the last two decades, from lung cancer to certain kinds of leukemia. But cancers of the same type aren’t all caused by the same mutations. Doctors must look for specific mutations before giving a targeted drug. If someone whose cancer doesn’t harbor the target mutation received such a treatment, it would expose them to the potential side effects without the benefits.
Standard biopsies to look for such mutations involve taking a tissue sample from tumors that can’t be removed surgically. This requires the insertion of a needle into a tumor to remove a small piece of tissue for analysis.
Because of these limitations, liquid biopsy tests have been developed that use blood samples to look for known cancer-causing mutations in ctDNA. Although ctDNA is relatively scarce in the bloodstream, Goel explained, that isn’t a problem when doctors already know exactly what mutation they’re looking for.
After a tumor has already been removed, ctDNA can also be used to track whether a cancer has come back after treatment. A tumor’s ctDNA can be detected long before the tumor could be picked up on standard imaging scans. For this approach, tumor tissue removed during surgery is used to create an individual ctDNA “signature” of that cancer. Periodic blood tests can then provide an early alert for the cancer’s return.
“We use liquid biopsies quite a bit now for monitoring recurrence and relapse,” Goel said.
Colorectal cancer #
Researchers are testing whether liquid biopsies can also help guide initial treatment. For example, in recent studies, scientists have been able to measure ctDNA to identify whether some people with colorectal cancer still have cancer cells in their body after surgery.
Studies are now looking at whether using this information can improve survival. One large NIH-funded clinical trial is testing whether people with colorectal cancer who receive chemotherapy guided by their liquid biopsy results live longer.
This is the knowledge gap that needs to be filled before these tests become widely used to guide treatment, Sorg explained. Research has shown that results from liquid biopsies are reproducible and reliable, he says. “Now, can we use this information to benefit patients? If we act on this information, do we see an increase in survival? Or an increase in quality of life?”
Quality of life #
Quality of life, including a reduction in the side effects from treatment, is the ultimate measure for many patients. So studies are also testing whether liquid biopsy can pick out people at low risk of cancer recurrence, who can safely skip treatments like chemotherapy without impacting their survival.
“If we can show with liquid biopsies that we can avoid treating patients at low risk of recurrence, then we can spare them the resulting side effects,” Sorg said.
Images #
Many thanks Olena, Jonathan Bailey, NHGRI, Miroslaw Miras!